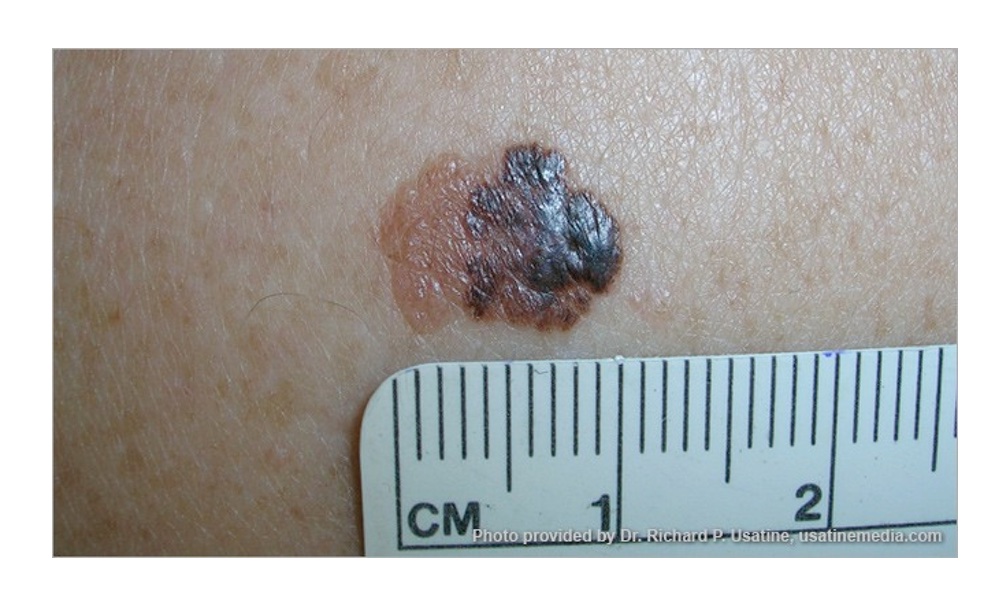
Malignant melanoma is the most invasive skin cancer with the highest risk of death. While it's a serious skin cancer, it's highly curable if caught early. Prevention and early treatment are critical, especially if you have fair skin, blonde or red hair and blue eyes.
The standard treatment for stage III melanoma, cancer that has spread to nearby lymph nodes or tissue but not to distant organs, is to first surgically remove the tumor, including any affected lymph nodes, and after surgery treat the patient with a combination of immunotherapy, targeted therapy and, sometimes, radiation therapy to suppress any remaining cancerous cells.
Researchers at M.D. Anderson Cancer Center wanted to see what might happen if this sequence were reversed, if the patient had surgery after these other treatments have been applied.
Their reasoning was this: “If immunotherapy eliminates most of the tumor before surgery, then we have sufficiently trained the immune system for an antitumor response, which minimizes the possibility of recurrence,” explained Elizabeth Burton, executive director of the University of Texas MD Anderson's Strategic Research Initiative Development (STRIDE) program, in a statement.Eighty percent of the patients enrolled in the study had no recurrence of their cancer four years later, a significant milestone.
The team tried this approach on 30 melanoma patients. Their goal was to shrink the melanoma tumor and prime a patient's immune system before surgery. Four years later, 80 percent of the patients enrolled in the study had no recurrence of their cancer, a significant milestone.
Stage III melanoma has a high risk of recurrence following surgery, and the researchers hoped that the new immunotherapies, by fighting against the melanoma, might make the subsequent surgery more effective. This was not a wild guess or shot in the dark. Earlier results had shown that these new therapies, the checkpoint inhibitors nivolumab and relatlimab, were very effective after surgery.
The newer drug, relatlimab, is a LAG-3 inhibitor, that was approved in 2022 in combination with nivolumab by the Food and Drug Administration (FDA) for patients with advanced melanoma. Relatlimab is designed to block a protein that sits on T cells, an important white blood cell that helps the body attack cancer cells. This particular protein normally acts as a brake, stopping the T cell from going too far, but here, for the treatment of melanoma, scientists wanted the anti-cancer T cells to work overtime. One way to do that, they hoped, is to cut the brakes.Scientists wanted the anti-cancer T cells to work overtime to stop malignant melanoma, so they cut the brakes on T cells.
The study also showed that patients who had high pre-treatment levels of one biomarker, called TIGIT, or low levels of another biomarker, called B7-H3, had the best chance of remaining recurrence-free, highlighting the potential to use these markers to predict patient responses in the future.
Going forward, the authors are collaborating with other researchers at MD Anderson's James P. Allison Institute to validate these biomarkers and to use spatial profiling to further understand where they are located and how they can impact the tumor microenvironment.
The study is published in the Journal of Clinical Oncology.