Dr. Orenstein is Professor of Medicine and Pediatrics, Emory University School of Medicine, and was formerly Director, National Immunization Program of the Centers for Disease Control and Prevention (CDC).
Dr. Orenstein has been a consultant to Chiron, has received research funds from the Chiron Foundation and has previously received research funds from Medimmune.
Dr. Orenstein has been a consultant to Chiron, has received research funds from the Chiron Foundation and has previously received research funds from Medimmune.
Few infectious disease burdens can compare with the impact of global influenza pandemics. During the past century, the United States was affected by three pandemics, 1918-19, 1957-58, and 1968-69 which accounted for approximately 500,000, 70,000, and 34,000 deaths, respectively. In addition, pandemic influenza can be associated with severe strains on the nation's health care delivery capacity as well as substantial economic disruptions. The Department of Health and Human Services has estimated (Table 1) the health burden of a moderate and a severe pandemic in the United States.
Table 1.
Number of Episodes of Illness, Healthcare Utilization and Death Associated with Moderate and Severe Pandemic Influenza Scenarios.*
| Characteristic | Moderate (1958/68-like) | Severe (1918-like) |
|---|---|---|
| Illness | 90 million (30%) | 90 million (30%) |
| Outpatient medical care | 45 million (50%) | 45 million (50%) |
| Hospitalization | 865,000 | 9,900,000 |
| ICU care | 128,750 | 1,485,000 |
| Mechanical ventilation | 64,875 | 742,500 |
| Deaths | 209,000 | 1,903,000 |
*Estimates based on extrapolation from past pandemics in the United States. Note that these estimates do not include the potential impact of interventions not available during the 20th century pandemics.
Source: www.pandemicflu.gov, p18.
Source: www.pandemicflu.gov, p18.
Overall, approximately 30% of the population would develop clinical disease, approximately 50% of these persons would seek medical care and up to 1.9 million would die of their illness. Reports of human cases of an avian influenza virus, H5N1, in at least seven countries since 2003, raise concerns that a new pandemic may be at hand. While most influenza experts believe a pandemic of influenza is inevitable, it is not clear when the pandemic will strike, which virus will be its cause and how severe the health burden will be. However, it is important to enhance our preparedness now to be ready for the next pandemic, whenever it should occur.
The Influenza Virus
The influenza virus consists of eight segmented genes.
Figure 1.
Influenza Virus.
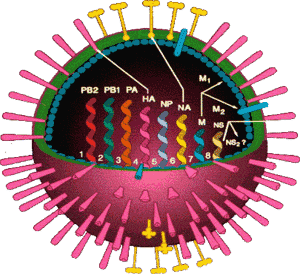
Cartoon of the influenza virus with its eight segmented genes and two critical surface glycoproteins, the hemagglutinin (HA) and the neuraminidase (NA).
There are two sugar-based proteins (glycoproteins), the hemagglutinin (H) and the neuraminidase (N), which project from the virus surface and play a critical role in the disease process. The hemagglutinin attaches to receptors on the host cell surface thus allowing the virus to enter5 The neuraminidase is important in final packaging and release of virus from infected cells and may be important in disease severity.
There are two major types of viruses associated with human disease, influenza A and influenza B. Influenza B viruses are stable, that is, their mutations are usually small, they don't mutate into completely new versions, and are not associated with pandemics. Influenza B viruses tend to cause epidemics of influenza which vary in magnitude, severity and interval in years from prior epidemics.
Influenza A viruses are associated with both annual epidemics and global pandemics. There are 16 known hemagglutinins of the influenza A virus, H1 - H16 and nine neuraminidases, N1-N9. Water fowl, particularly ducks and geese, are the natural hosts for the virus. Only three hemagglutinins are known to have circulated in humans — H1, H2 and H3. The H1N1 virus circulated from 1918-19 until 1957 when it was replaced by the A/Asian, H2N2 virus. This virus continued to circulate until 1968 when an H3N2 virus became predominant. In 1977, an H1 virus returned, which was similar to earlier strains of H1N1. This latter strain primarily affects young persons since persons born prior to 1957 were likely exposed to a similar virus and are usually immune. At the present time, three types of influenza viruses circulate in humans, A/H3N2 which tends to be responsible for the most severe of the annual epidemics, A/H1N1 and B. The strains that predominate in a given year vary.There are two major types of viruses associated with human disease, influenza A and influenza B.
Antigenic drift occurs when the entire hemagglutinin is replaced by a completely new type of hemagglutinin to which virtually the entire population is susceptible. The new hemagglutinins are believed to originate in avian species. For example, the replacement of H1 viruses by H2 viruses in 1957 represented an antigenic shift. There are three criteria which must be met for a pandemic to occur:
- an antigenic shift takes place creating a virus against which almost the entire population lacks immunity,
- the virus causes serious human disease and
- the virus is transmitted efficiently from human to human.
The second mechanism involves mutation of avian viruses which make them efficient in both infecting humans and being transmitted from human to human. Such mutations account for the adaptation of the 1918-19 A/H1N1 virus to humans.Antigenic drift occurs when the entire hemagglutinin is replaced by a completely new type of hemagglutinin to which virtually the entire population is susceptible.
Avian Influenza
While there are many avian strains of influenza, only a limited number have been associated with human disease. Recently, there have been transmissions of H9 and H7 viruses to humans. These infectious have been limited and illnesses have frequently been mild. However, of greatest concern have been the H5N1 viruses. The first documented human cases arising from H5N1 viruses occurred in Hong Kong during 1997. During the outbreak, 18 cases were reported resulting in six deaths. Blood samples at the time among household, workplace and health care worker contacts showed there may have been very limited human-to-human transmission.
Between 1998 and 2002, there were a few isolated poultry outbreaks in Hong Kong. However, by late 2003, H5N1 viruses had become responsible for a massive infection of avian influenza in East Asia fowl. Furthermore, the virus had mutated and whereas it had previously caused mild illnesses in chickens, it was now causing severe disease and death.
As of February 13, 2006, the H5N1 virus had spread through migratory birds throughout East Asia, into West Asia, Eastern and Western Europe, and several countries of Africa. There was concern because of bird migration during the winter that the virus would be introduced into Africa. Indeed, WHO is now reporting poultry cases in northern Nigeria (www.who.int/csr/don/2006_02_08/en/). The greater the dissemination of virus, the greater is the chance for mutation and reassortment, which could result in a human-adapted pandemic strain.
Epidemiology of Avian Influenza
The reservoir of the virus is aquatic fowl. The virus may be excreted from healthy-appearing or sick birds in their oral and respiratory secretions as well as in stool. The virus can survive in droppings and aerosols of such droppings can be infectious.
The major risk factor is exposure to sick poultry, although, because the virus can cause infection in birds without any symptoms, some persons will not have such exposures. Persons of all ages can develop severe disease but most cases reported to date have been young. In contrast, annual influenza epidemics, which may cause substantial disease particularly in school-aged children, tend to cause their greatest mortality in the elderly.
A major epidemiologic study in Vietnam reported that persons with direct contact with sick or dead poultry in households were 1.73 times as likely to develop a flu-like illness as persons living in households without such contact.
Individuals will usually develop the disease 2-4 days after exposure, although intervals can range up to eight days. Most patients with influenza are shedding virus up to a day before onset of symptoms and are most contagious during the first 1-3 days of illness.
Some small outbreaks suggest person to person transmission may have occurred. The best documented of these occurred in Thailand where an 11-year-old girl apparently infected a 26-year-old mother and a 32-year-old aunt without known chicken exposure. Both secondary cases had provided extensive unprotected nursing care to the 11-year-old girl. However, to date, the vast majority of cases can be linked to poultry exposure as the source rather than transmission from an infected person.
Human Cases of Avian Influenza
Clinical Characteristics
As of February 13, 2006, the World Health Organization has received reports of 169 confirmed human cases of H5N1 avian influenza and 91 deaths from seven countries: Vietnam, Thailand, Cambodia, Indonesia, China, Turkey and Iraq (www.who.int/csr/disease/avian_influenza/country/cases_table_2006_02_13/en/). Reports of 41 cases from Thailand, Vietnam and Cambodia during 2004 and 2005 have been used to evaluate the clinical characteristics.11 Most of the cases occurred in young people with median ages ranging from 14-22 years with a range of 2-58 years. Exposure to ill poultry was reported in 75-100% (Table 2). All of the cases had fever and almost all had cough. In contrast, to strains of human adapted influenza, which tend to cause other upper respiratory symptoms and, except for children, do not cause substantial gastrointestinal symptoms, avian influenza cases in several studies were not associated with runny nose (rhinorrhea) and were frequently linked to diarrhea and abdominal pain.
Table 2.
Clinical Characteristics of Confirmed Cases of Avian Influenza Reported from Thailand and Vietnam 2004 and Ho Chi Minh City and Cambodia 2005 (n=41 cases).*
| Median or Mean Range on % of Patients | |
|---|---|
| Age (yrs) | 13.7 - 22 years |
| Time from illness onset to presentation at hospital | 6 - 8 days (3/4 studies) |
| % Fever >38°C | 100% |
| Myalgia | 0 - 53% (3/4 studies) |
| Diarrhea | 41 - 70% |
| Abdominal pain | 24 - 50% (2/4 studies) |
| Vomiting | 0 - 24% (3/4 studies) |
| Cough | 94 - 100% |
| Rhinorrhea | 0 - 53% (3/4 studies) |
*Source: N Engl J Med 2005; 353: 1374-1385.
Pulmonary complications were common including shortness of breath, pulmonary infiltrates, and respiratory failure (Table 3).
Table 3.
Clinical Characteristics of Confirmed Cases of Avian Influenza Reported from Thailand and Vietnam 2004 and Ho Chi Minh City and Cambodia 2005 (n=41 cases).*
| Median or Mean Range on % of Patients | |
|---|---|
| Shortness of breath | 76 - 100% (3/4 studies) |
| Pulmonary infiltrates | 100% |
| Lymphopenia | 33 - 80% (3/4 studies) |
| Thrombocytopenia | 33 - 50% (3/4 studies) |
| Increased aminotransferase | 67 - 83% (3/4 studies) |
| Respiratory failure | 70 - 100% |
| Renal dysfunction | 10 - 24% (3/4 studies) |
*Source: N Engl J Med 2005; 353: 1374-1385.
A decrease in infection-fighting white cells, platelets (which help clotting), inflammation of the liver and problems with kidney funtion were frequently reported with avian flu.
Most cases tended to have prolonged courses prior to hospitalization, which usually occurred 6-8 days after onset.
Diagnosis
If you have an unexplained acute respiratory illness, particularly with lower respiratory tract involvement (e.g., shortness of breath), and you have traveled in the 7-14 days prior to onset to a country experiencing an avian influenza epidemic among birds, you should talk to your doctor immediately. Information on the current situation can be obtained from the World Health Organization website (www.who.int/csr/disease/avian_influenza/en/). Suspicion of avian influenza should be raised when the traveler had exposure within several feet of live or dead domestic fowl, wild birds or ducks. Unprotected contact with a person with suspected avian influenza should also raise suspicion.
Causes of Death
The major causes of death from avian influenza are an overwhelming viral pneumonia and an acute respiratory distress syndrome (ARDS). Other causes of death from seasonal influenza can include secondary bacterial infections, worsening of underlying illnesses, inflammation of the brain or the heart.
Treatment
Antiviral drugs
There are two major categories of antiviral drugs — the adamantanes (amantadine, rimantadine) and the neuraminidase inhibitors (oseltamivir, zanamivir). Most H5N1 viruses are resistant to the adamantanes so such agents should not be used for either therapy or prophylaxis.
Initial human isolates of H5N1 viruses have been susceptible to the neuraminidase inhibitors. However, their effectiveness, the dosages required and the length of treatment needed are not known at this time. Based on experience with treatment and prevention with human-adapted influenza strains, it is reasonable to provide equivalent treatment courses.
Oseltamivir, which is administered orally, has led to reductions in length of illness of 1-2 days in healthy adults and 1.5 days in children 1-12 years.18 Middle ear infections were reduced in children by 44%. Treatment should start within 48 hours of onset of illness and ideally the earlier that doses can be administered the better. Adults should be treated with 75 mg twice a day for five days. Doses for children one year of age or older are weight adjusted. Doses need to be reduced for patients in kidney failure.
Zanamivir is administered intranasally, 10 mg (two inhalations) twice a day for five days. In contrast to oseltamivir, which is licensed for treatment of persons one year of age or older, zanamivir is not licensed for persons less than seven years of age.Treatment should start within 48 hours of onset of illness and ideally the earlier that doses can be administered the better.
Recent reports raise concerns about the effectiveness of oseltamivir against H5N1 influenza viruses. Two persons were reported from Vietnam who developed resistance while on therapy and died still virus positive after the usual course of treatment had ended. Resistance to oseltamivir has been reported in Japan among children, many of whom received less than the recommended doses. However, there has not been evidence to date of transmission from human to human of resistant viruses. Experiments in mice suggest that recent H5N1 viruses are more infective than earlier viruses isolated from Hong Kong and that an eight-day regimen of oseltamivir provided significantly more benefit than the standard five-day course.
Some authorities have recommended that zanamivir be considered as an important alternative to oseltamivir.18 Resistance to this drug has not been a problem to date and from a molecular perspective changes to the virus that would be needed to make it zanamivir resistant would likely decrease its infectivity for humans. However, other experts are concerned that because H5N1 viruses may invade other tissues besides the lungs, zanamivir, as administered intranasally, may not achieve the blood levels needed to treat a body-wide virus when it has already been established but may be useful in preventing infection in the first place.
For the present, your doctor should treat a suspected case of avian influenza with a five-day course of oseltamivir, ideally starting the drug within 48 hours of onset. The WHO website (www.who.int/csr/disease/avian_influenza/en/) and the CDC website (www.cdc.gov/flu/avian/) should be monitored for potential changes to the treatment recommendations.
Other Treatment Issues
Patients with avian influenza should be isolated for at least seven days after their fever breaks and possibly up to 21 days. If available, they should be placed in negative pressure rooms (where air flows into the room but not out) to prevent cross-contamination. Staff should wear N-95 respirators, gowns, goggles and gloves. Visitors should be restricted.
Exposed health care workers should monitor their temperature twice a day. If fever develops, they should be evaluated and, if no alternative cause is identified, should be placed on a neuraminidase inhibitor. Health care workers exposed to potentially infectious aerosols as a result of a lapse in technique should be considered for a 7-10 day course of prophylactic treatment with neuraminidase inhibitor (75 mg per day).
Household contacts of avian influenza cases should also be monitored for fever and, if febrile, should be treated with a neuraminidase inhibitor.
Measures to Control Spread
Vaccines
Influenza vaccines are the optimal means of preventing influenza. Two vaccines are available to prevent annual influenza pandemics — an inactivated vaccine administered by injection and a live attenuated (i.e., weakened), cold adapted, temperature sensitive vaccine (LAIV) which is administered via the nose (intranasally). The inactivated vaccine is most commonly used and is 70-90% effective against influenza among healthy adults when the strains in the vaccine and the circulating strains are similar. Effectiveness among the elderly, particularly the frail elderly, is substantially less against illness but the vaccine tends to be more effective against complications of influenza leading to hospitalization and death. LAIV is highly effective in young children but at the moment has very limited indications, primarily for persons 5-49 years of age without conditions that place them at high risk of complications from influenza.
The major problems with vaccines against pandemic strains are:
- the time interval needed from detection of the virus to production of the first doses (generally at least four months);
- limited production capacity within the United States (only one manufacturer produces vaccine solely within the US and only about five million standard potency doses would be expected weekly after the minimum four-month production time);
- a two-dose schedule, one month apart, is likely to be needed;
- the quantity of antigen needed in each dose may be up to six times the standard dose (90 mcg versus 15 mcg) and
- virus mutations mean vaccines produced against today's strains may be ineffective against strains that actually cause the epidemic.
- moving production from the current manufacture in embryonated chicken eggs to cell-based production to be in a better position to meet surge demands and decrease production time;
- testing of adjuvants to decrease the amount of antigen needed per dose and potentially reduce the number of doses needed;
- evaluating alternative forms of delivery, such as intradermal vaccination, to decrease the amount of antigen needed per dose; and
- assuring that there is year-round egg supply to quicken reaction time when new strains are detected.
Because vaccine supply may be short of demand, the Advisory Committee on Immunization Practices (ACIP) and the National Vaccine Advisory Committee (NVAC) have developed a list, in priority order, of persons for whom vaccines would be targeted. This list is based on maintaining the critical infrastructure, especially of the health care delivery system, as well as protecting those persons thought to be most vulnerable to complications. The actual priorities would have to be adjusted based on the epidemiology of the pandemic strain. The list of priorities is shown in Table 4.
Table 4.
Vaccine Priority Groups.
| Element and Tier | Personnel (1000's) | Cumulative Total (1000's) |
|---|---|---|
| 1A. Health care involved in direct patient contact + essential support | 9,000 | 9,000 |
| Vaccine and antivirals manufacturing personnel | 40 | 9,040 |
| 1B. Highest risk group | 25,840 | 34,880 |
| 1D. Key government leaders + critical public health pandemic responders | 151 | 45,731 |
| 2. Rest of high risk | 59,100 | 104,831 |
| Most CI and other PH emergency responders | 8,500 | 113,331 |
| 3. Other key government health decision makers + mortuary services | 500 | 113,831 |
| 4. Healthy 2-64 years not in other groups | 179,260 | 293,091 |
Source: Department of Health and Human Services: HHS Pandemic Influenza Plan, November 2005; available at www.pandemicflu.gov, pp D-10 to D-29.
Antivirals
The NVAC has called for a national stockpile of neuraminidase inhibitors sufficient to treat approximately 25% of the population. The NVAC has also established a list, in priority order, of persons who should be treated if they have avian influenza or who should receive prophylaxis. The focus of treatment is on hospitalized patients. The focus on prophylaxis is toward critical health care delivery infrastructure. The NVAC priorities are shown in Table 5.
Table 5.
| Target Group | Estimated Population (millions) | Strategy | Target Group | Cumulative |
|---|---|---|---|---|
| # Courses (in millions) | ||||
| Patients admitted to hospital | 10.0 | T | 8.0 | 8.0 |
| HCWs with direct patient contact | 9.2 | T | 2.4 | 10.4 |
| Highest risk outpatients | 2.5 | T | 0.7 | 11.1 |
| Pandemic health responders, pub safety & key gov decision makers | 3.3 | T | 0.9 | 12.0 |
| Increased risk outpatients | 85.5 | T | 22.4 | 34.4 |
| Outbreak response | N/A | PEP | 2.0 | 36.4 |
| HCWs in ER, ICU, EMS, dialysis | 1.2 | P | 4.8 | 41.2 |
| Pandemic societal responders & other HCWs | 10.2 | T | 2.7 | 43.9 |
| Other outpatients | 180 | T | 47.3 | 91.2 |
| Highest risk outpatients | 2.5 | P | 10.0 | 101.2 |
| Other HCWs w/ patient contact | 8.0 | P | 32.0 | 133.2 |
Source: Department of Health and Human Services: HHS Pandemic Influenza Plan, November 2005; available at www.pandemicflu.gov, pp D-10 to D-29.
Other Prevention Measures
The World Health Organization has recently reviewed a variety of non-pharmaceutical interventions to stem the spread of pandemic influenza. These include isolation of cases, quarantine of contacts, social distancing (e.g., school closing, avoiding crowding), use of masks in public, and hygiene and disinfection. The potential impact of these measures will depend upon the actual characteristics of the pandemic strain including its infectiousness, communicability during the asymptomatic incubation period, transmission from persons with asymptomatic infection, duration of infectiousness, and the primary age groups participating in dissemination. Based on information, primarily from annual epidemics, peak infectivity would be expected early during the symptomatic phase of the illness.
Effectiveness of non-pharmaceutical measures will also depend upon the stage of the pandemic. If transmission is already widespread, internationally and nationally, isolation and quarantine are less likely to have an impact compared to earlier stages. Such measures may be most important to implement when the pandemic is first detected, transmission is limited and new introductions of the virus from outside would be unlikely.
Isolation is designed to prevent transmitting cases from coming in contact with susceptible individuals. Quarantine applies to isolating the exposed but well contacts of cases, potentially in the incubation period, to prevent their contact with other persons. While isolation of cases early in a pandemic seems reasonable, mandatory quarantine of contacts may be difficult to enforce and carries with it obligations to provide food and shelter. It is not clear whether such a strategy given the short incubation period and doubling time of cases would be effective.
On the other hand, it appears reasonable to call for voluntary social distancing. With a severe pandemic, it is likely that the population will stop venturing out from their homes except for necessities, such as food and medical care, and voluntary calls for stopping social gatherings, such as sporting events, will be heeded. School closures may be more effective in rural than urban settings because of the greater potential for urban children to have contact outside of the school system but may be important in both settings if children are primary transmitters.
Wearing of masks in public needs further evaluation since its effectiveness is not clear. Handwashing and cough etiquette seem reasonable but their effectiveness for influenza are not known.
What Can Be Done in the Interpandemic Period to Enhance Preparedness?
The timing, severity and characteristics of a future pandemic are unknown. However, there are a number of steps that can be taken to enhance preparedness. An elaboration of many of these steps is available at the official government pandemic influenza website (www.pandemicflu.gov). Some measures apply specifically to pandemic influenza. Others may be useful for all types of disasters.
Since vaccine is the best means of preventing influenza, actions that would enhance the vaccine production and delivery infrastructure should be undertaken. For example, influenza vaccine is recommended annually for approximately 180 million Americans, yet the greatest number of doses ever distributed has been only 83 million. Efforts should be made to vaccinate a greater proportion of persons for whom influenza vaccine is already recommended (Table 6). This will encourage manufacturers to enhance capacity and be in a better position to make large quantities of vaccines more rapidly. In addition, the capacity to deliver vaccines to large populations rapidly will be increased. A focus on improving routine influenza vaccination has immediate benefits, regardless of whether or not a pandemic occurs in the near future.
Table 6.
Annual Influenza Immunization Recommendations.
People at high risk of severe illness if they get influenza
People who can give the flu to those at high riskPeople 50-64 years old
Anoyone who wants to prevent the flu
Establishment of antiviral stockpiles at the federal and state level would help in assuring supply is adequate to meet the needs. Institutions may also consider stockpiling however individual stockpiles are discouraged.
- 65 and older
- Adults and children with a chronic health condition
- Children 6-23 months old
- Women who will be pregnant during the flu season
- Residents of long-term care facilities
- Persons with disorders that compromise respiratory function, handling respiratory secretions or increase aspiration
People who can give the flu to those at high risk
- Household member or caregiver of someone at high risk
- Health care workers
- Household member or caregiver of a child under two years old
Development and refinement of state, local and institutional plans can be useful to address issues such as staffing, security and minimization of economic disruptions.
Summary
Future influenza pandemics are a certainty. However, the timing of such pandemics, the influenza virus or viruses that will cause them and the severity are unknown. An unprecedented outbreak of avian influenza A/H5N1, now affecting poultry in many countries throughout Asia, Europe and Africa and the occurrence of severe human cases in seven countries, primarily from exposure to poultry, has raised concerns that the H5N1 virus may become the next pandemic strain. Whether or not the H5N1 virus is the next pandemic strain, its occurrence provides impetus for improving our preparedness.
Preparedness starts with improving surveillance to detect potential pandemic strains as quickly as possible to provide lead time for development of vaccines and implementation of other measures. Clinical suspicion of avian influenza should be raised when someone with unexplained acute respiratory illness is evaluated with a history of recent travel to an area where there are avian or human cases. Obtaining laboratory confirmation is critical and physicians should contact local health authorities immediately when they suspect a case. Vaccines are the best means of reducing influenza and its complications but may not be available early in a pandemic. The initial viruses isolated to date have been susceptible to neuraminidase inhibitors (oseltamivir and zanamivir) and although oseltamivir resistance has been reported, it is reasonable to consider both drugs for treatment and prophylaxis. Other measures such as isolation, social distancing, use of masks and respiratory hygiene may be of some benefit in slowing the epidemic and in buying time for vaccine production and delivery. An important step in preparing for a pandemic is to increase the vaccine production capacity and delivery infrastructure for annual influenza vaccination since it is this capacity that will be needed to produce and deliver pandemic vaccines. Less than 50% of Americans, for whom influenza vaccine is recommended annually, are vaccinated.
ADVERTISEMENT




